在随机对照试验(Randomized Controlled Trial,RCT)中,非药物RCT的干预措施大多涉及多种复杂的治疗手段,很难进行统一、规范、重复和实施。实施因素的差异、治疗者的专业知识水平和治疗中心的医疗水平等均会影响试验干预效果的评估。据此,CONSORT小组制定了非药物治疗随机对照试验(Nonpharmacologic Treatments Randomized Control Trials)报告的统一标准(Consolidated Standards of Reporting Trials,CONSORT)扩展版。本文概述非药物随机对照临床试验CONSORT扩展版相关内容。
关键词:随机对照试验; 报告规范; 临床试验报告的统一标准; CONSORT; 非药物干预临床试验; 非药物治疗临床试验; CONSORT扩展版
一、非药物治疗随机对照试验报告规范背景
随机对照试验(Randomized Controlled Trial,RCT)的干预措施可以是药物,也可以是非药物,后者主要包括手术、技术干预、仪器设备干预、康复理疗、心理治疗、行为干预、补充和替代医学疗法等。近年来,非药物RCT数量逐渐增加,但CONSORT声明标准版尚未考虑非药物RCT的特殊性,如其干预措施大多是涉及多种复杂的治疗手段,很难统一、规范、重复和实施;难以实施盲法,即使实施,方法及设计也较为复杂;结果易受到实施因素的差异、治疗者的专业知识水平和治疗中心的医疗水平等各种因素影响,进而影响非药物RCT报告的质量,有偏地评估干预效果。
基于此,CONSORT小组制定了非药物干预临床试验的报告规范Extending the CONSORT Statement to Randomized Trials of Nonpharmacologic Treatment,即《非药物随机对照临床试验CONSORT扩展声明》,并于2006年2月在法国巴黎召开会议就如何报告非药物干预的RCT达成了共识。2008年,非药物随机对照临床试验的CONSORT扩展声明正式发表。2009年我国将此声明译为中文并推荐使用。非药物随机对照临床试验CONSORT扩展声明一共有22项,其中第1、3、4、7、8、11、12、13、20、21条内容根据非药物随机对照临床试验的特点进行了补充,另外新增1条内容,并对流程图进行了修改,其他项目与CONSORT 2001标准版相同。
二、非药物随机对照临床试验CONSORT扩展版条目清单及流程图
(一) 非药物随机对照临床试验CONSORT扩展版条目清单
表1 非药物治疗随机对照试验CONSORT扩展版的条目清单中英文对照
| 报告项目 | 条目编号 | CONSORT标准版内容 | 非药物临床试验扩展版内容 |
| Title and abstract 标题与摘要 | 1 | How participants were allocated to interventions (e.g.,"random allocation" "randomized", or "randomly assigned") 如何把患者分配到不同的干预组(如“随机分配”“随机化”) | In the abstract, description of the experimental treatment, comparator, care providers, centers, and blinding status 在摘要中,指出试验组与对照组的干预手段、医疗保健提供者、试验中心以及设盲方法 |
| Introduction 引言 | |||
| Background 背景 | 2 | Scientific background and explanation of rationale 论述研究背景和解释相关原理 | |
| Methods 方法 | |||
| Participants 研究对象 | 3 | Eligibility criteria for participants, settings and locations where the data were collected 报告纳入标准和数据收集的环境和地点 | When applicable, eligibility criteria for centers and those performing the interventions 必要时,指出试验中心和干预措施执行者的入选标准 |
| Interventions 干预措施 | 4 | Precise details of the interventions intended for each group and how and when they were actually administered 准确描述每组的干预,以及实施干预的过程和时间 | Precise details of both the experimental treatment and comparator 详细介绍试验组与对照组的干预措施 |
| 4a | Description of the different components of the interventions and, when applicable, descriptions of the procedure for tailoring the interventions to individual participants 描述实施干预的各个步骤,必要时,描述针对个别受试者而对干预步骤所作的修改 | ||
| 4b | Details of how the interventions were standardized 规范干预手段的细节 | ||
| 4c | Details of how adherence of care providers with the protocol was assessed or enhanced 评估或提高医疗保健提供者对研究方案依从性的细节 | ||
| Objectives 目的 | 5 | Specific objectives and hypotheses 明确说明目标和假设 | |
| Outcomes 结局 | 6 | Clearly defined primary and secondary outcome measures and, when applicable, any methods used to enhance the quality of measurements (e.g., multiple observations, training of assessors) 清楚定义主要结局和次要结局,如果可以的话,还应报告提高结局测量质量的方法(如多次测量,测量者培训) | |
| Sample size 样本量 | 7 | How sample size was determined and, when applicable, explanation of any interim analyses and stopping rules 报告如何确定样本量,可以的话还应该报告中期分析结果和终止试验的规则 | When applicable, details of whether and how the clustering by care providers or centers was addressed 必要时,详述医疗保健提供者和试验中心是否以及如何进行群组分配 |
| Randomization sequence generation 随机分配序列的产生 | 8 | Method used to generate the random allocation sequence, including details of any restriction (e.g., blocking, stratification) 报告产生随机分配序列的方法,包括任何限制性随机化的细节(如区组、分层) | When applicable, how care providers were allocated to each trial group 在适当的情况下,介绍医疗保健提供者如何分配至各试验组 |
| Allocation concealment 分配隐匿 | 9 | Method used to implement the random allocation sequence (e.g., numbered containers or central telephone), clarifying whether the sequence was concealed until interventions were assigned 报告实施随机的方法(如编码的容器或中心电话),明确在实施干预之前是否进行分配隐藏 | |
| Implementation 随机化实施 | 10 | Who generated the allocation sequence, who enrolled participants, and who assigned participants to their groups 说明由谁生成随机分配序列,谁招募研究对象,谁对研究对象进行分组 | |
| Blinding(masking) 盲法 | 11a | Whether or not participants, those administering the interventions, and those assessing the outcomes were blinded to group assignment 是否对受试者、干预实施者和结局评估者设盲 | Whether or not those administering co-interventions were blinded to group assignment 是否对实施共同干预措施的人员设盲 |
| 11b | If blinded, method of blinding and description of the similarity of interventions 如果进行了设盲,说明如何设盲,以及干预措施的相似之处 | ||
| Statistical methods 统计学方法 | 12 | Statistical methods used to compare groups for primary outcome(s); and methods for additional analyses, such as subgroup analyses adjusted analyses 说明比较主要结局指标的统计方法;其他分析方法,如亚组分析和调整分析 | When applicable, details of whether and how the clustering by care providers or centers was addressed 必要时,详述医疗保健提供者和试验中心是否以及如何进行组群分配 |
| Results 结果 | |||
| Participant flow 研究对象纳入流程 | 13 | Flow of participants through each stage (a diagram is strongly recommended). Specifically, for each group report the numbers of participants randomly assigned, receiving intended treatment, completing the study protocol, and analyzed for the primary outcome. Describe protocol deviations from study as planned, together with reasons 报告每一阶段参与试验的研究对象人数(强烈建议使用流程图)——即每个组的随机分配的人数、接受治疗的人数、完成治疗方案的人数、用于主要结局分析的人数等。对于违背研究计划的地方,还要对其原因加以解释 | The number of care providers or centers performing the intervention in each group and the number of patients treated by each care provider or in each center 各组医疗保健提供者或施行干预的试验中心的数目,每个医疗保健提供者或每个试验中心治疗的患者数目 |
| Implementation of intervention 干预的实施 | New item 新增 | Details of the experimental treatment and comparator as they were implemented 试验组和对照组实施干预的细节 | |
| Recruitment 招募 | 14 | Dates defining the periods of recruitment and follow-up 说明招募和随访的日期 | |
| Baseline data 基线数据 | 15 | Baseline demographic and clinical characteristics of each group 描述基线时每组的人口统计学资料和临床特征 | When applicable, a description of care providers (case volume, qualification, expertise, etc.) and centers (volume) in each group 必要时,介绍每组的医疗保健提供者(诊治病例数、资格和专业知识等)以及试验中心(包括中心数目) |
| Numbers analyzed分析例数 | 16 | Number of participants (denominator) in each group included in each analysis and whether the analysis was by "intention-to-treat". State the results in absolute numbers when feasible (e.g., 10/20, not 50%) 每项分析中都需要报告每组分析的研究对象人数(分母),注明是否采用了意向性分析;如果可能,应该采用绝对数报告结果(例如10/20,而非50%) | |
| Outcomes and estimation 结局和效应估计 | 17 | For each primary and secondary outcome, a summary of results for each group, and he estimated effect size and its precision (e.g., 95% confidence interval) 对于每一项主要结局和次要结局,需报告每组的结果、效应值及其精确度(如95%置信区间) | |
| Ancillary analyses 其他分析 | 18 | Address multiplicity by reporting any other analyses performed, including subgroup analyses and adjusted analyses, including which are prespecified and which are exploratory 若处理多因素,应报告其他分析,包括亚组分析和调整分析,并指出哪些是事先设定的,哪些是探素性的 | |
| Adverse events 不良事件 | 19 | All important adverse events or side effects in each intervention group 报告每个组发生的所有重要的不良事件或副作用 | |
| Discussion 讨论 | |||
| Interpretation 解释 | 20 | Interpretation of the results, taking into account study hypotheses, sources of potential bias or imprecision, and the dangers associated with multiplicity of analyses and outcomes 解释结果时要考虑研究假设、潜在的偏倚或不精确的原因,以及多重比较和多种结局的问题 | In addition, take into account the choice of the comparator, lack of or partial blinding, and unequal expertise of care providers or centers in each group 此外,还应该考虑对照的选择,未实施盲法或者部分设盲,以及各组卫生保健提供者或试验中心专业技能不均等的问题 |
| Generalizability 外推性 | 21 | Generalizability (external validity) of the trial findings 解释试验结果的外推性(外部真实性) | Generalizability (external validity) of the trial findings according to the intervention, comparators, patients, and care providers and centers involved in the trial 依据试验中涉及的干预措施、对照措施、患者以及医疗保健提供者和医疗保健中心得出的结果的可推广性(外部有效性) |
| Overall evidence 整体证据 | 22 | General interpretation of the results in the context of current evidence 基于当前证据对结果进行概括性解释 | |
(二) 非药物随机对照临床试验CONSORT声明报告流程图
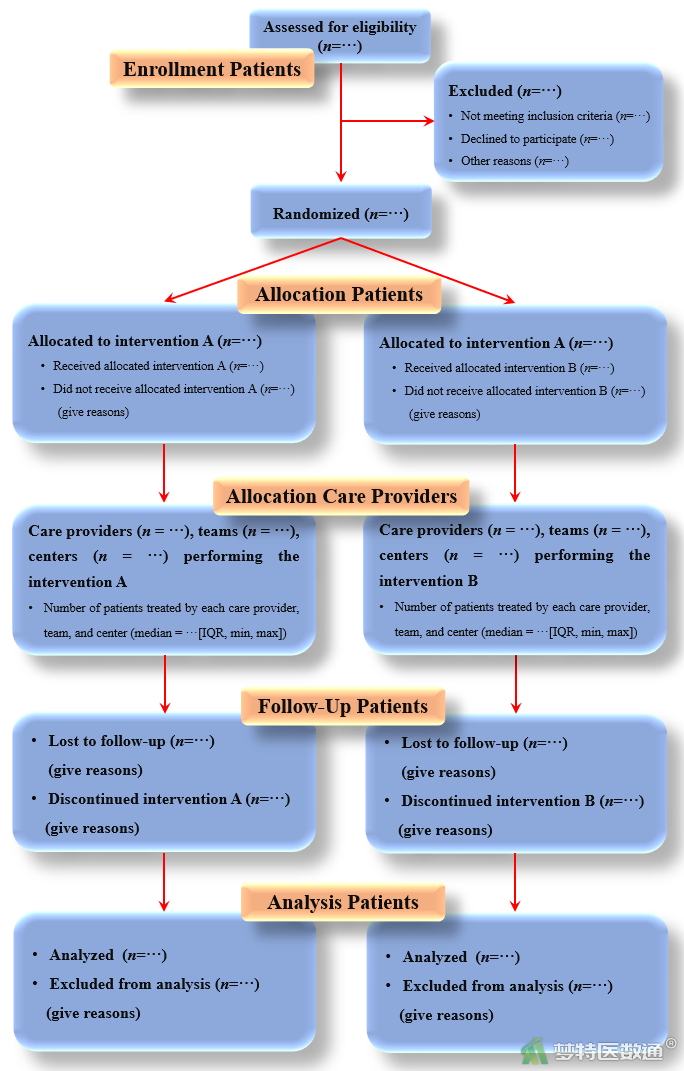
图1 非药物随机对照临床试验CONSORT声明流程图(英文)
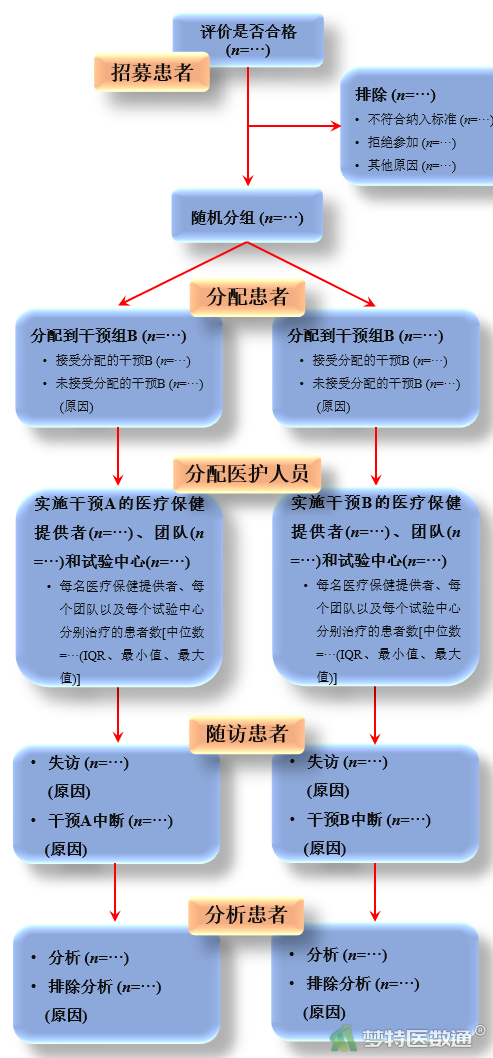
图2 非药物随机对照临床试验CONSORT声明流程图(中文)
三、非药物随机对照临床试验CONSORT扩展版使用注意事项
非药物随机对照临床试验CONSORT扩展版是CONSORT标准版的扩展声明之一,旨在提高非药物随机对照临床试验报告的透明度。该指南既保留了平行设计RCT的条目,也突出了非药物随机对照临床试验自身的特点。该扩展版意味着作者、编辑和审稿人认为:一份阐明方法学并不完善的透明报告比缺乏透明度而掩盖可能不完善方法的研究更容易发表。
尽管非药物随机对照临床试验CONSORT扩展版并不是为了改善RCT的计划和实施,但作者在试验计划书的撰写和试验开展阶段阅读该扩展版,并以此为依据解释和说明文件评估非药物随机对照临床试验的结果以及撰写论文,可提高报告质量;编辑和审稿人在评议此类稿件时,也可参考该扩展版。
研究者在使用本条目清单中的22个条目时,应该将该清单内容与CONSORT 2010标准版指南结合起来。根据实施的试验类型和研究内容不同,作者也可以与整群试验和非劣效性与等效性试验所制定的CONSORT声明扩展版,以及不良事件报告的详细指南联合使用。
注:本文内容是参考相关文献后对非药物治疗随机对照试验CONSORT扩展版原文的概述,仅代表本网站观点。关于非药物治疗随机对照试验CONSORT扩展版的更多内容详见官方网站(http://www.consort-statement.org)或论文Methods and Processes of the CONSORT Group: Example of an Extension for Trials Assessing Nonpharmacologic Treatments (https://pubmed.ncbi.nlm.nih.gov/18283201/)、报告非药物随机对照临床试验的CONSORT扩展声明:说明与详述(一)( https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e75TZJapvoLK3O-LTbfNYgMEWngAW9eWew4h3g4g_631fOKEgfM_G7fjMZxfS-tuX8&uniplatform=NZKPT)、报告非药物随机对照临床试验的CONSORT扩展声明:说明与详述(二)( https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e75TZJapvoLK3O-LTbfNYgMKBpFQrwNCsIqsY-0ygu-AyVTdqjoBz99cs9Kcm_z2U3&uniplatform=NZKPT)、报告非药物随机对照临床试验的CONSORT扩展声明:说明与详述(三)( https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e75TZJapvoLK3O-LTbfNYgMGI_WPMBiJ1f07Rc6gHxv2UsS95qB51aVjjlQDnEg2Vx&uniplatform=NZKPT)。